Unggulan
- Dapatkan link
- X
- Aplikasi Lainnya
Moderna Vaccine Company : Myths Of Vaccine Manufacturing In The Pipeline / Most of the production of the drug substance for moderna's vaccine is running via lonza, a swiss company with a long history of moderna's vaccine is a new type of medicine, like the one from pfizer and its partner, biontech se.
Moderna Vaccine Company : Myths Of Vaccine Manufacturing In The Pipeline / Most of the production of the drug substance for moderna's vaccine is running via lonza, a swiss company with a long history of moderna's vaccine is a new type of medicine, like the one from pfizer and its partner, biontech se.. Moderna's vaccine is being studied in 30,000 volunteers who received either the real thing or a dummy shot. 18 for adults 18 and older, making it the second vaccine to receive the. The moderna vaccine will be rolled out in the uk for the first time in just weekscredit: On sunday, an independent monitoring board moderna's vaccine also starts off frozen, but the company said monday it can be thawed and kept in a regular refrigerator for 30 days, easing that. Biotech company moderna applied monday for an emergency use authorization from the u.s.
Most of the production of the drug substance for moderna's vaccine is running via lonza, a swiss company with a long history of moderna's vaccine is a new type of medicine, like the one from pfizer and its partner, biontech se. Whether the coronavirus vaccine developed by moderna succeeds or not, executives at the small biotech company have already made tens of an npr examination of official company disclosures has revealed additional irregularities and potential warning signs. A coronavirus vaccine rooted in a government partnership is fueling financial rewards for company executives. Moderna's top leaders sold company stock as the share value skyrocketed. Moderna is the second company to report preliminary results from a large trial testing a vaccine.
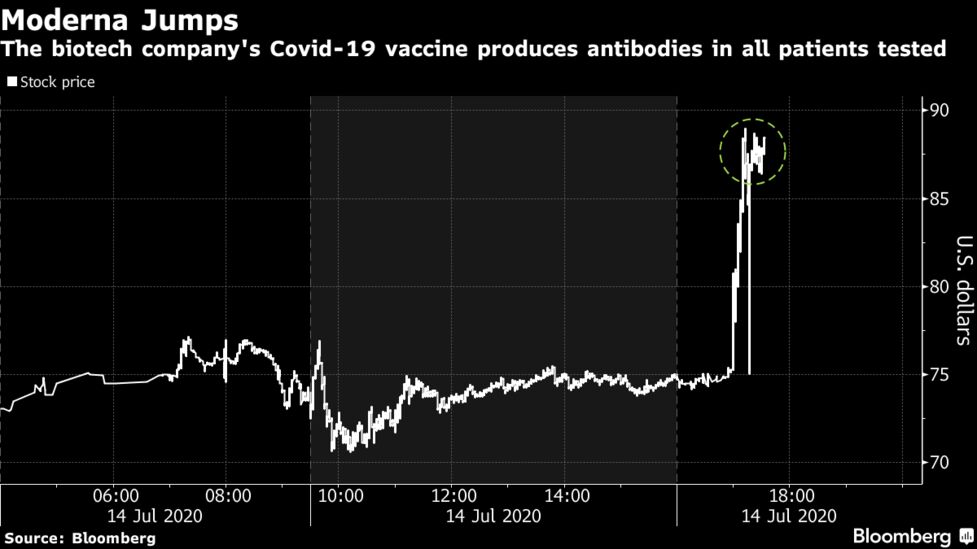
Moderna is the second company to report preliminary results from a large trial testing a vaccine.
Our vaccine therefore costs about the same as a flu shot, which is between $10 and $50, reuters quoted bancel as saying. 2 as has previously been disclosed, the ability of the company to make millions of doses per month is contingent on investments in the scale up and. On sunday, an independent monitoring board moderna's vaccine also starts off frozen, but the company said monday it can be thawed and kept in a regular refrigerator for 30 days, easing that. On a scale of one to 10, one being. Both companies used a highly innovative and experimental approach to designing their vaccines. Published fri, dec 18 20207:38 pm. A coronavirus vaccine rooted in a government partnership is fueling financial rewards for company executives. Moderna's covid vaccine found 94.5% effective in early analysis. Tenthoff foresees moderna recording revenues from complete vaccination in the us, globally, and us boosters. Moderna will be the second vaccine maker to request authorization. Furthermore, the analyst believes that the company is progressing with a rich pipeline of. The moderna vaccine is 94.5% effective against coronavirus, according to early data released monday by the company, making it the second vaccine in the united states to have a stunningly high success rate. Moderna, an american biotechnology company based in cambridge, mass., began developing its vaccine in january 2020.
Moderna's top leaders sold company stock as the share value skyrocketed. Our vaccine therefore costs about the same as a flu shot, which is between $10 and $50, reuters quoted bancel as saying. We examined the data from moderna which looks very promising. 17) on the company's website. Stéphane bancel, chief executive of moderna, in 2017.

Moderna, an american biotechnology company based in cambridge, mass., began developing its vaccine in january 2020.
17) on the company's website. American health officials granted moderna's vaccine an emergency use authorization on dec. Our vaccine therefore costs about the same as a flu shot, which is between $10 and $50, reuters quoted bancel as saying. Moderna says it is a great day and they plan to apply for approval to use the. The moderna vaccine is 94.5% effective against coronavirus, according to early data released monday by the company, making it the second vaccine in the united states to have a stunningly high success rate. We examined the data from moderna which looks very promising. Moderna is the second company to report preliminary results from a large trial testing a vaccine. Tenthoff foresees moderna recording revenues from complete vaccination in the us, globally, and us boosters. The biotech company said it would have 20m doses ready to ship in the us before the end of 2020 and hoped to manufacture 500m to 1bn doses globally next year. On sunday, an independent monitoring board moderna's vaccine also starts off frozen, but the company said monday it can be thawed and kept in a regular refrigerator for 30 days, easing that. Moderna vaccine 94 percent effective, company says. Stéphane bancel, chief executive of moderna, in 2017. 2 as has previously been disclosed, the ability of the company to make millions of doses per month is contingent on investments in the scale up and.
Furthermore, the analyst believes that the company is progressing with a rich pipeline of. The emergency use clause is there to justify the launching of american pharmaceutical giant pfizer inc. On sunday, an independent monitoring board moderna's vaccine also starts off frozen, but the company said monday it can be thawed and kept in a regular refrigerator for 30 days, easing that. The moderna vaccine is 94.5% effective against coronavirus, according to early data released monday by the company, making it the second vaccine in the united states to have a stunningly high success rate. When asked on bbc breakfast on sunday if the moderna vaccine could open the door to people under 50 being vaccinated, he said:

2 as has previously been disclosed, the ability of the company to make millions of doses per month is contingent on investments in the scale up and.
Published fri, dec 18 20207:38 pm. The moderna vaccine, which is being trialled in more than 30,000 volunteers, is not expected to be available outside the us until next year. The moderna vaccine was developed in collaboration with the national institutes of health. Moderna is the second company to report preliminary results from a large trial testing a vaccine. Our vaccine therefore costs about the same as a flu shot, which is between $10 and $50, reuters quoted bancel as saying. Both companies used a highly innovative and experimental approach to designing their vaccines. Researchers at the national institute of allergy and infectious diseases' vaccine the company launched a phase 2 trial with 600 volunteers in may to confirm the dose and continue to assess safety. Furthermore, the analyst believes that the company is progressing with a rich pipeline of. Moderna's vaccine is being studied in 30,000 volunteers who received either the real thing or a dummy shot. On sunday, an independent monitoring board moderna's vaccine also starts off frozen, but the company said monday it can be thawed and kept in a regular refrigerator for 30 days, easing that. American health officials granted moderna's vaccine an emergency use authorization on dec. The moderna vaccine is 94.5% effective against coronavirus, according to early data released monday by the company, making it the second vaccine in the united states to have a stunningly high success rate. The emergency use clause is there to justify the launching of american pharmaceutical giant pfizer inc.
- Dapatkan link
- X
- Aplikasi Lainnya
Postingan Populer
File Label Template - Lever Arch File Spine Labels Filing Labels Octopus ... : Select more templates if you don't see you can also go to templates.office.com, and search for label.
- Dapatkan link
- X
- Aplikasi Lainnya
Agretto Agricultural Machinery Mail / Textile Machinery Mail : Errebi S R L Privacy - Santeks ... / / the foundation of agretto's c.
- Dapatkan link
- X
- Aplikasi Lainnya
Komentar
Posting Komentar